
The morphology of nanoscale materials such as the size and the shape of the nanoparticles and nanocrystals can dramatically affect their properties. Research on nanoscale materials is motivated by the observation that materials, previously only known in their bulk phase, show a significant change in physical and chemical properties or even exhibit novel phenomena at the nanoscale due to a high surface-to-volume ratio and finite size effects. The thorough characterization and understanding of these properties in interplay with nanoscopic length scales will ultimately guide the way to the exploitation of these effects in applications, including high density storage media and biomedical materials. The group of nanoscale chemistry is focusing on the synthesis of inorganic, organic, and hybrid nanomaterials. For instance, we develop new methods to synthesize single-crystalline inorganic nanowires and nanocrystals directly inside the carbon nanotubes. On even a smaller scale, single metal ions and small clusters are encapsulated inside fullerenes during their formation in arc-discharge synthesis forming endohedral fullerenes. Carbon nanostructures in such hybrid materials act not only as templating matrices but also as protecting shields stabilizing the nanosized forms of inorganic materials. Unique electronic, transport, and magnetic properties of these hybrid heterostructures are achieved due to nanosize of encapsulated structures and the interface effects at the boundary with the carbon π-system are then studied in close cooperation with other groups of IFW. Reducing only one dimension to nanoscopic length scale we have to consider ultrathin layers of the materials. The charge carrier densities of ultrathin layers which are part of an electric double layer can be influenced strongly by applying an electrochemical potential. Our aim is to investigate how huge charge carrier densities will influence the electric and magnetic properties of appropriate materials.
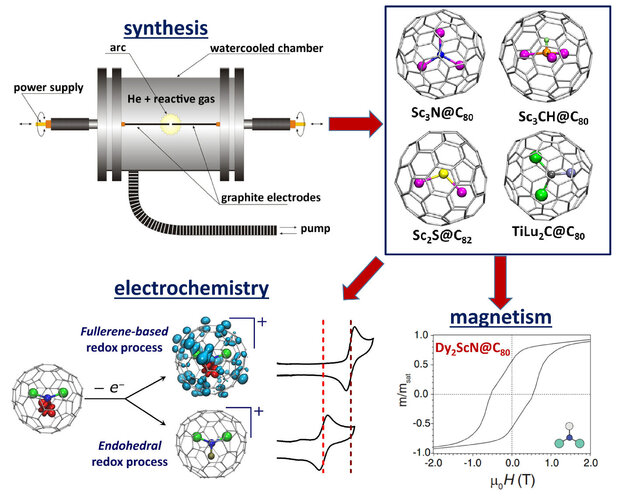
The empty space inside the fullerene cage can be filled with atom, ions, clusters, or even small molecules. The fullerenes with encapsulated species are called endohedral fullerenes. Particular focus of our work is the synthesis of endohedral metallofullerenes (EMFs) with different clusters. The group in IFW Dresden pioneered in the development of the reactive atmosphere method, in which NH3 or CH4 gases are used as a source of nitrogen or hydrogen in the arc-discharge synthesis of EMF. More recent developments include the use of solid nitrogen-containing organic compounds as the sources of nitrogen. Reaction atmosphere method results in the dramatic suppression of the empty fullerene formation, leading to EMFs as the main fullerene products. We are also looking for new types of EMFs, which led to the discovery of such clusterfullerenes as Sc3CH@C80, Sc2S@C82, or TiLu2C@C80. Encapsulation of metal atoms within the carbon-based π-system results in a variety of unprecedented chemical and physical properties of EMFs. In IFW Dresden we are specifically focused on the electron-transfer mechanism in EMFs as studied by electrochemistry and spectroelectrochemistry, and on the magnetic properties of lanthanide-based EMFs, including their single molecule magnetism. Experimental studies are accompanied by quantum-chemical calculations of molecular structure, spectroscopic properties, and spin states.
Group Leader: Dr. Alexey Popov
Phone: +49 351 4659 871
Email
Syhthesis of EMFs and single-crystal X-ray diffraction: Dr. Fupin Liu
Phone: +49 351 4659 1130
Email
Computational studies of static and dynamic properties: Dr. Stanislav Avdoshenko
Phone: +49 351 4659 1130
Email
Dr. Alexey A. Popov (Group leader)
Dr. Fupin Liu, head of the group "Synthesis and properties"
Dr. Stanislav Avdoshenko, head of the group"Computational studies"
PhD Students
Yaofeng Wang
Synthesis of EMFs and derivatives
Wei Yang
Synthesis of EMFs and derivatives
Vasilii Dubrovin
Quantum chemical calculations
Georgios Velkos
Magnetometry, XMCD
Emmanouil Koutsouflakis
Scanning tunneling microscopy
Technicians
Alex Beger
Sandra Schiemenz
Frank Ziegs
Recent Alumni and Visitors
Dr. Lukas Spree, PhD student (defense in July 2020)
Victor Nekrasov, visiting PhD student (Moscow State University, 2019)
Yajuan Hao, visiting PhD student (Soochow University, 2018-2019)
Dr. Peter Machata, Postdoc (till 09.2019)
Svetlana Sudarkova, visiting PhD student (Moscow State University, 2018 and 2019)
Jimmy Kuo, visiting student (2-months DAAD Fellowship in 2018)
Dr. Christin Schlesier, PhD student (defense in November 2018)
Dr. Ariane Brandenburg, PhD student (defense in September 2018)
Dr. Chia-Hsiang Chen, Postdoc (till 07.2018)
Dr. Denis Krylov, PhD student (defense in June 2017)
Dr. Nataliya Samoylova, PhD student (defense in September 2017)
Dr. Katrin Junghans, PhD student (defense in May 2017)
Dr. Qingming Deng, PhD student (defense in May 2016)
Metallofullerene photoswitches driven by photoinduced fullerene-to-metal electron transfer.
M. Zalibera, F. Ziegs, S. Schiemenz, V. Dubrovin, W. Lubitz, A. Savitsky, S. Deng, X.-B. Wang, S. Avdoshenko, A. A. Popov.
Chem. Sci.2021, 12, 7818-7838. DOI: 10.1039/D0SC07045A
Magnetic hysteresis at 10 K in single molecule magnet self-assembled on gold
C.-H. Chen, L. Spree, E. Koutsouflakis, D. S. Krylov, F. Liu, A. Brandenburg, G. Velkos, S. Schimmel, S. M. Avdoshenko, A. Fedorov, E. Weschke, F. Choueikani, P. Ohresser, J. Dreiser, B. Büchner, A. A. Popov.
Adv. Sci.2021, 8 (5), 2000777. DOI: 10.1002/advs.202000777
Caught in Phase Transition: Snapshot of the Metallofullerene Sc3N@C70 Rotation in the Crystal
Y. Hao, Y. Wang, V. Dubrovin, S. M. Avdoshenko, A. A. Popov, F. Liu.
J. Am. Chem. Soc.2021, 143 (2), 612–616.DOI: 10.1021/jacs.0c10758
Substrate‐independent magnetic bistability in monolayers of single molecule magnet Dy2ScN@C80 on metals and insulator
D. S. Krylov, S. Schimmel, V. Dubrovin, F. Liu, T. T. N. Nguyen, L. Spree, C.-H. Chen, G. Velkos, C. Bulbucan, R. Westerström, M. Studniarek, J. Dreiser, C. Hess, B. Büchner, S. M. Avdoshenko, A. A. Popov
Angew. Chem. Int. Ed. 2020, 59 (14), 5756-5764. DOI: 10.1002/anie.201913955
Air-stable redox-active nanomagnets with lanthanide spins radical-bridged by a metal-metal bond
F. Liu, G. Velkos, D. S. Krylov, L. Spree, M. Zalibera, R. Ray, N. A. Samoylova, C.-H. Chen, M. Rosenkranz, S. Schiemenz, F. Ziegs, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter, M. Richter, B. Büchner, S. M. Avdoshenko, A. A. Popov
Nat. Commun. 2019, 10, 571. DOI: 10.1038/s41467-019-08513-6
Single-electron lanthanide-lanthanide bonds inside fullerenes toward robust redox-active molecular magnets
F. Liu, L. Spree, D. S. Krylov, G. Velkos, S. M. Avdoshenko, A. A. Popov
Acc. Chem. Res. 2019, 52 (10), 2981-2993. DOI: 10.1021/acs.accounts.9b00373
Single molecule magnetism with strong magnetic anisotropy and enhanced Dy∙∙∙Dy coupling in three isomers of Dy-oxide clusterfullerene Dy2O@C82
W. Yang, G. Velkos, F. Liu, S. M. Sudarkova, Y. Wang, J. Zhuang, H. Zhang, X. Li, X. Zhang, B. Büchner, S. M. Avdoshenko, A. A. Popov, N. Chen
Adv. Sci. 2019, 1901352. DOI: 10.1002/advs.201901352
High blocking temperature of magnetization and giant coercivity in the azafullerene Tb2@C79N with a single-electron Tb–Tb bond
G. Velkos, D. S. Krylov, K. Kirkpatrick, L. Spree, V. Dubrovin, B. Büchner, S. M. Avdoshenko, V. Bezmelnitsyn, S. Davis, P. Faust, J. Duchamp, H. C. Dorn, A. A. Popov
Angew. Chem. Int. Ed. 2019, 58, 5891. DOI: 10.1002/anie.201900943
Thermally-activated delayed fluorescence in Y3N@C80 endohedral fullerene: time resolved luminescence and electron paramagnetic resonance studies
M. Zalibera, D. S. Krylov, D. Karagiannis, P.-A. Will, F. Ziegs, S. Schiemenz, W. Lubitz, S. Reineke, A. Savitsky, A. A. Popov
Angew. Chem. Int. Ed. 2018, 130 (1), 283–287. DOI: 10.1002/anie.201710637
Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih
D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Büchner, A. A Popov
Chem. Commun. 2017, 53, 7901-7904. DOI: 10.1039/C7CC03580B
Single molecule magnet with an unpaired electron trapped between two lanthanide ions inside a fullerene
F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner, A. A. Popov
Nat. Commun. 2017, 8, 16098. DOI: 10.1038/ncomms16098
Synthesis and Structure of LaSc2N@Cs(hept)‐C80 with One Heptagon and Thirteen Pentagons
Y. Zhang, K. B. Ghiassi, Q. Deng, N. Samoylova, M. M. Olmstead, A. L. Balch, A. A. Popov
Angew. Chem. Int. Ed. 2015, 54 (2), 495-499. DOI: 10.1002/anie.201409094
Perfluoroalkylfullerenes
O. V. Boltalina, A. A. Popov, I. V. Kuvychko, N. B. Shustova, S. H. Straus
Chem. Rev. 2015, 115, 1051-1105. DOI: 10.1021/cr5002595
Methane as a selectivity booster in the synthesis of endohedral fullerenes: towards selective synthesis of the single molecule magnet Dy2TiC@C80 and its congener Dy2TiC2@C80
K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerström, T. Greber, B. Büchner, A. A. Popov
Angew. Chem. Int. Ed. 2015, 54 (45), 13411-13415. DOI: 10.1002/anie.201505870
Endohedral fullerene with μ3-carbido ligand and Titanium-Carbon double bond stabilized inside a carbon cage
A. L. Svitova, K. B. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. M. Olmstead, A. L. Balch, L. Dunsch, A. A. Popov
Nat. Commun. 2014, 5, 3568. DOI: 10.1038/ncomms4568
Clusters encapsulated in Endohedral Metallofullerenes: How strained are they?
Q. Deng, A. A. Popov
J. Am. Chem. Soc. 2014, 136 (11), 4257-4264. DOI: 10.1021/ja4122582
Endohedral Fullerenes
A. A. Popov, S. Yang, L. Dunsch
Chem. Rev. 2013, 113 (8), 5989–6113. DOI: 10.1021/cr300297r
Bonding between strongly repulsive metal atoms: an oxymoron made real in a confined space of endohedral metallofullerenes
A. A. Popov, S. M. Avdoshenko, A. M. Pendás, L. Dunsch
Chem. Commun. 2012, 48, 8031-8050. DOI: 10.1039/C2CC32568C
Internal @ IFW Dresden
External
Scaling a material down to nanometer-size reveals several opportunities to increase physical properties compared to bulk material or even create new ones. When quantum effects come into play electrical conductivity, magnetic permeability and chemical reactivity change as a function of particle size. Our approach uses carbon nanotubes (CNT) as a reaction container for the synthesis of intermetallic nanoparticles. With the given diameter of the tube particle size becomes adjustable. Furthermore the presence of the CNT-surrounding eases the reduction to metallic particle and protects nanoparticles from chemical influences (e.g. oxidation). Depending on the used material one obtains single particles or nano wires inside the inner cavity of the CNT. CNT have been filled for example with elements of main group IV in their metallic state. 3 different procedures to post synthetically fill the inner cavity of the CNT were used and by variation of the reaction parameters the appearance of filling particles, degree of filling and the purity of the samples in terms of coating to filling particle ratio can be tailored. These filled CNT with high rates of filling are promising for sensoric and energy storage applications.
The interest on 2D materials is rapidly growing and chemical approaches offer absolute control over the structure of 2D materials at the molecular-level. The chemical approach will serve as strategy to develop new multifunctional systems, featuring exceptional physical or chemical properties with optimal control over the correlation between structure and function. Our research currently focusses on the chemical vapor transport (CVT) in sealed ampules. One example is the CVT of bismuth chalcogenides nanostructures (Bi2Ch3; Ch = S, Se, Te) which can be synthesized by catalyst-free decomposition sublimation. The nanostructures directly grow on Si/SiO2 substrates by a vapor−solid growth mechanism and show high degree of crystallinity with dimensions of >10 μm in length and simultaneously <10 nm in height (nanoribbons). In order to optimize the growth process in a reproducible way we realize parallel thermodynamic calculations. The electrical transport data are evidence that this approach offers the chance to synthesize and investigate crystals with high quality and to measure surface state properties.
HeatCNT
In this EFRE (European Regional Development Fund) project, the IFW Dresden and the Fraunhofer-Institut für Fertigungstechnik u. Angewandte Materialforschung, develop a composite material made of carbon nanotubes (CNTs) in a copper matrix for application as a heat-exchange medium. By playing with different fabrication parameters such as the alignment of the CNTs, their volume fraction, and selective etching that leaves CNTs partially exposed, it is possible to tailor the thermal properties of the material for specific radiative, convective and conducting applications.
Schlott A, Hutsch T, Hampel S, Lohse J, Weißgärber T, Kieback B. Heat Exchange Structures Based on Copper/CNT Composite. Key Engineering Materials 2019;809:106–14. https://doi.org/10.4028/www.scientific.net/kem.809.106
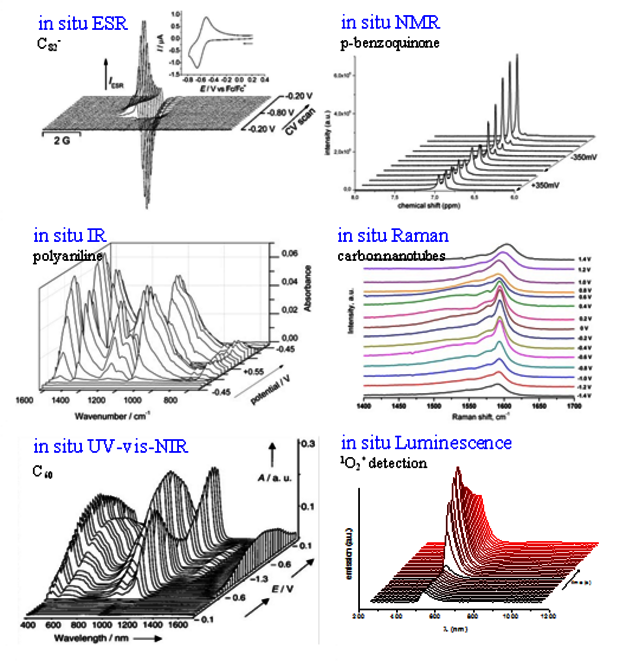
Spectroelectrochemistry as the combination of electrochemistry and various spectroscopic methods delivers detailed information on the electron transfer reactions in organic semiconductors:
(i) detection of electrochemically generated species;
(ii) their characterization (structure, properties, stability);
(iii) concentration profile of charge carriers in dependence of applied potential;
(iv) elucidation of the electrochemical reaction mechanism.
We are studing π-conjugated organic structures (small organic molecules, oligomers, conducting polymers etc.), carbon nanostructures (nanotubes, empty and endohedral fullerenes, and their functional derivatives etc.), hybrid molecular structures (metal-organic compounds, coordination complexes, endohedral metallofullerenes etc.), reactive oxygen species (ROS) in solution.
We have an expertise in various electrochemical and spectroscopic techniques and their combinations for in situ measurements:
M. Rosenkranz, S. Leßny, B. Noecker, S. Breakspear, E. Dmitrieva
Formation of free radicals in human hair under strain: Combined electron paramagnetic resonance (EPR) – Strain technique
Talanta | 2022 | Volume: 249 | P. 123707 | URL
E. Dmitrieva, M. Rosenkranz, Y. Alesanco, A. Viñuales
Spectroelectrochemical study of alkyl-aryl asymmetric viologens in poly(vinyl alcohol) (PVA) – borax electrolyte
Electrochimica Acta | 2018 | Volume: 323 | P. 134792 | URL
E. Dmitrieva, M. Rosenkranz, J.S. Danilova, E.A. Smirnova, M.P. Karushev, I.A. Chepurnaya, A.M. Timonov
Radical formation in polymeric nickel complexes with N2O2 Schiff base ligands: An in situ ESR and UV-vis-NIR spectroelectrochemical study
Electrochimica Acta | 2019 | Volume: 283 | P. 1742-1752 | URL
J. Danilova, E. Dmitrieva, S. Avdoshenko, I. Chepurnaya, M. Karushev, A. Timonov
Infrared spectral features of the charge carriers in nickel salen polymeric complexes
Electrochimica Acta | 2024 | Volume: 492 | P. 144387 | URL
The Spectroelectrochemistry group in IFW has state-of-the-art equipment and expertise for in situ studies of the electrochemical electron transfer in soluiton using IR, Raman, ESR, NMR, UV-vis-NIR and luminescence techniques.
Bruker EMXplus X-band CW spectrometer with a variable temperature unit and an integrated device for the EPR-strain measurements
Avantes diode array spectrometer (200 - 2000 nm)
Bruker FTIR spectrometer Vertex 80v (50-8000 cm-1, vacuum)
confocal FTIR microscope Hyperion 2000
Bruker Avance II 500 MHz with a standard 5 mm probe head
Thermo Fisher Scientific DXR Smart Raman spectrometer (532, 633 and 780 nm laser excitation)
Advanced Research Raman System T64000 (Horiba Scientific) with confocal Raman microscope
potentiostats (HEKA, Metrohm Autolab, PAR)
UV-Vis-NIR spectrophotometer (UV-3600i Plus, Shimadzu) with multi-purpose large-sample compartment and integrating sphere attachment (220 - 2600 nm)